High definition 3-Bromopropylamine Hydrobromide - Eptifibatide for Treatment of Acute Coronary Syndrome 188627-80-7 – Gentolex
High definition 3-Bromopropylamine Hydrobromide - Eptifibatide for Treatment of Acute Coronary Syndrome 188627-80-7 – Gentolex Detail:
Product Detail
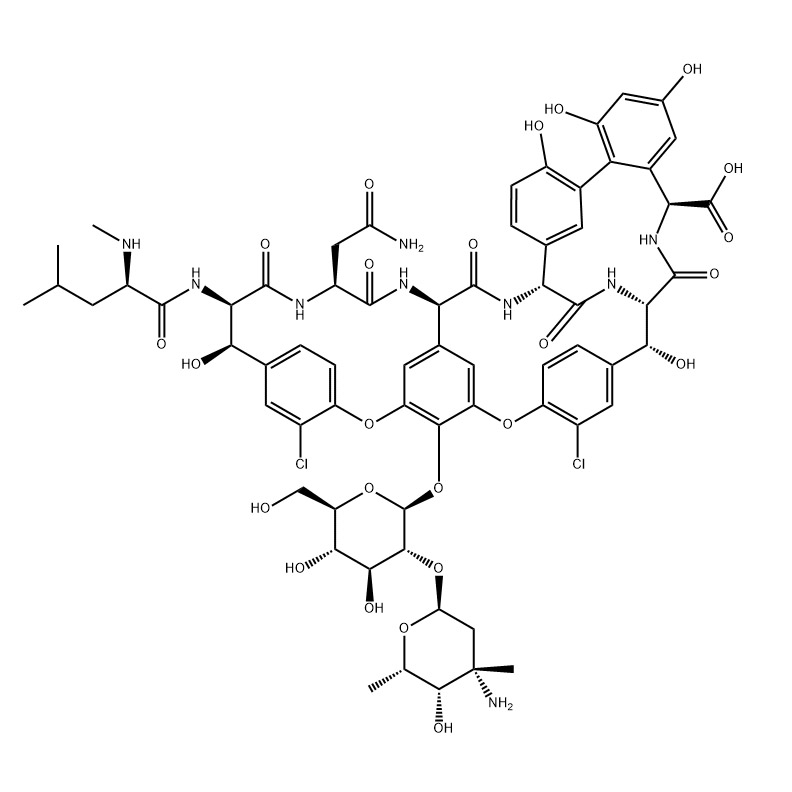
| Name | Eptifibatide |
| CAS number | 188627-80-7 |
| Molecular formula | C35H49N11O9S2 |
| Molecular weight | 831.96 |
| EINECS Number | 641-366-7 |
| Density | 1.60±0.1 g/cm3(Predicted) |
| Storage conditions | Sealed in dry, store in freezer, under -15°C |
Synonyms
Eptifibatideacetatesalt;Eptifibatide,MPA-HAR-Gly-Asp-Trp-Pro-Cys-NH2,MPAHARGDWPC-NH2,>99%;MAP-LYS-GLY-ASP-TRP-PRO-CYS-NH2;INTEGRELIN;Eptifibatide;N6-(Aminoiminomethyl)-N2-(3-mercapto-1-oxopropyl-L-lysylglycyl-L-a-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide;MPA-HAR-GLY-ASP-TRP-PRO-CYS-NH2(DISULFIDEBRIDGE,MPA1-CYS6).
Platelet Glycoprotein Antagonist
Etifibatide (integrilin) is a novel polypeptide platelet glycoprotein IIb/IIIa receptor antagonist, which inhibits platelet aggregation and thrombosis by inhibiting the last common pathway of platelet aggregation. Compared with the monoclonal antibody abciximab, eptifibatide has a stronger, more directional and specific binding to GPIIb/IIIa due to the existence of a single conservative amino acid substitution—lysine to replace arginine. Therefore, it should have a good therapeutic effect in the interventional treatment of acute coronary syndrome. Platelet glycoprotein IIb/IIIa receptor antagonist drugs have been developed a lot, and currently there are 3 kinds of preparations that can be used in clinical internationally, abciximab, eptifibatide and tirofiban. ). There is little experience in the use of platelet glycoprotein GPIIb/IIIa receptor antagonists in China, and the available drugs are also very limited. Only one drug, tirofiban hydrochloride, is on the market. Therefore, a new platelet glycoprotein IIb was developed. /IIIa receptor antagonists are imperative. Domestic eptifibatide is an imitation product produced by Chengdu Sino Biological Products Co., Ltd.
Classification
Classification of Antiplatelet Aggregation Drugs
Antiplatelet aggregation drugs can be roughly divided into three categories: 1. Cyclooxygenase-1 (COX-1) inhibitors, such as aspirin. 2. Inhibit platelet aggregation induced by adenosine diphosphate (ADP), such as clopidogrel, prasugrel, cangrelor, ticagrelor, etc. 3. Platelet glycoprotein Ⅱb/Ⅲa receptor antagonists, such as abciximab, eptifibatide, tirofiban, etc. In addition, there are prostaglandin EP3 receptor inhibitors, newly synthesized chemical components and effective extracts from traditional Chinese medicine.
Product detail pictures:


Related Product Guide:
We've been commitment to offer the competitive rate ,outstanding merchandise good quality, too as fast delivery for High definition 3-Bromopropylamine Hydrobromide - Eptifibatide for Treatment of Acute Coronary Syndrome 188627-80-7 – Gentolex , The product will supply to all over the world, such as: Jamaica, British, venezuela, As a way to use the resource on the expanding info in international trade, we welcome prospects from everywhere on the web and offline. In spite on the high quality items we offer, effective and satisfying consultation service is supplied by our qualified after-sale service group. Item lists and detailed parameters and any other info weil be sent to you timely for the inquiries. So please make contact with us by sending us emails or call us when you've got any questions about our organization. ou could also get our address information from our site and come to our enterprise. We get a field survey of our merchandise. We are confident that we'll share mutual accomplishment and create solid co-operation relations with our companions within this market place. We're seeking forward for your inquiries.
This supplier's raw material quality is stable and reliable, has always been in accordance with the requirements of our company to provide the goods that quality meet our requirements.