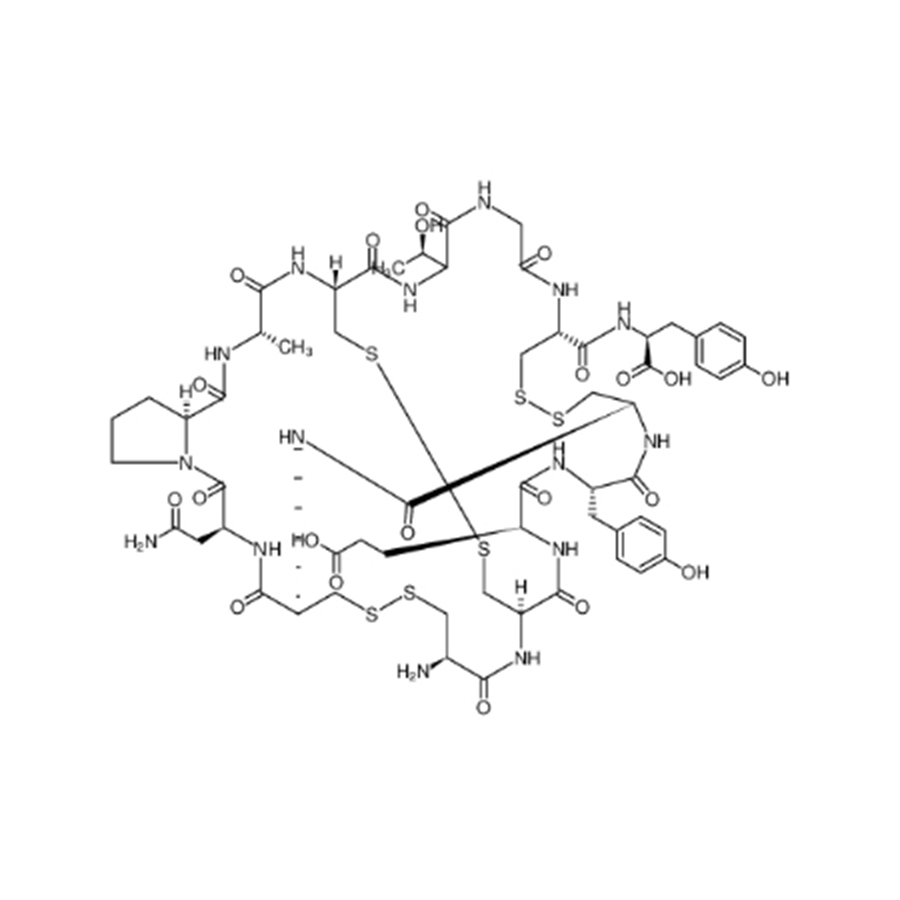
OEM Supply Sunitinib Malate Intermediate - Desmopressin Acetate to Treat central Diabetes Insipidus – Gentolex
OEM Supply Sunitinib Malate Intermediate - Desmopressin Acetate to Treat central Diabetes Insipidus – Gentolex Detail:
Product Detail
| Name | Desmopressin |
| CAS number | 16679-58-6 |
| Molecular formula | C46H64N14O12S2 |
| Molecular weight | 1069.22 |
| EINECS Number | 240-726-7 |
| Specific rotation | D25 +85.5 ± 2° (calculated for the free peptide) |
| Density | 1.56±0.1 g/cm3(Predicted) |
| RTECS No. | YW9000000 |
| Storage conditions | Store at 0°C |
| Solubility | H2O:soluble20mg/mL, clear, colorless |
| Acidity coefficient | (pKa) 9.90±0.15 (Predicted) |
Synonyms
MPR-TYR-PHE-GLN-ASN-CYS-PRO-D-ARG-GLY-NH2; MINIRIN; [DEAMINO1, DARG8] VASOPRESSIN; [DEAMINO-CYS1, D-ARG8]-VASOPRESSIN; DDAVP, HUMAN; DESMOPRESSIN; DESMOPRESSIN, HUMAN; DESAMINO-[D-ARG8] VASOPRESSIN
Indications
(1) Treatment of central diabetes insipidus. After the drug can reduce urinary excretion, reduce urinary frequency and reduce nocturia.
(2) Treatment of nocturnal enuresis (patients aged 5 years or older).
(3) Test the renal urine concentration function, and carry out the differential diagnosis of renal function.
(4) For hemophilia and other bleeding diseases, this product can shorten the bleeding time and prevent bleeding. It can reduce the amount of intraoperative blood loss and postoperative oozing; especially in conjunction with reasonably controlled blood pressure during surgery, it can reduce intraoperative bleeding from different mechanisms, and reduce postoperative oozing, which can play a better role in blood protection.
Treatment of diabetes insipidus
Diabetes insipidus is primarily a disorder of water metabolism characterized by excess urine output, polydipsia, hypoosmolarity, and hypernatremia. Partial or complete deficiency of vasopressin (central diabetes insipidus), or renal insufficiency of vasopressin (nephrogenic diabetes insipidus) can be onset. Clinically, diabetes insipidus is similar to primary polydipsia, a condition in which excessive fluid intake is caused by a malfunction of the regulatory mechanism or abnormal thirst. Contrary to primary polydipsia, the increase in water intake in patients with diabetes insipidus is a corresponding response to changes in osmotic pressure or blood volume.
Product detail pictures:


Related Product Guide:
As for competitive selling prices, we believe that you will be searching far and wide for anything that can beat us. We will state with absolute certainty that for such excellent at such charges we are the lowest around for OEM Supply Sunitinib Malate Intermediate - Desmopressin Acetate to Treat central Diabetes Insipidus – Gentolex , The product will supply to all over the world, such as: Panama, Salt Lake City, Curacao, We adopted technique and quality system management, based on "customer orientated, reputation first, mutual benefit, develop with joint efforts", welcome friends to communicate and cooperate from all over the world.
The accounts manager made a detailed introduction about the product, so that we have a comprehensive understanding of the product, and ultimately we decided to cooperate.